Determine the Molarity of a Solution
It is used to calculate the volume of solvent or the amount of solute. Molarity is used to measure the concentration of a solution.
6 1 Calculating Molarity Problems Chemistry Libretexts
The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume.

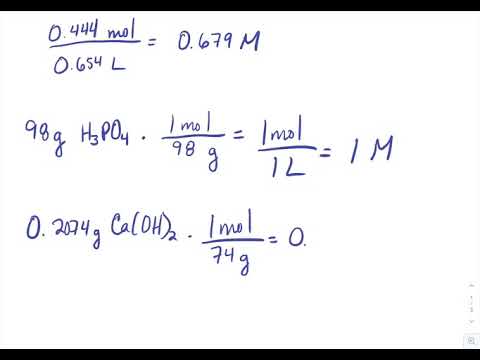
. Determine the molarity for each of the following solutions02074 g of calcium hydroxide CaOH2 in 4000 mL of solutionOpenStax is a registered trademark. Find the molarity of a sugar solution given the grams of solute sucrose dissolved the volume of solution and the molecular mass of sucrose 3422965 grams per mole. Mass of a compound required to prepare a solution of known volume and concentration.
If youre seeing this message it means were having trouble loading external resources on our website. 28 Determine the molarity of a solution formed by dissolving 977 g LiBr in enough water to yield 7500 mL of solution. This problem has been solved.
Therefore the molality of solution is 333 mol kg. A solution of ethanol in water in which the mole fraction of ethanol is 0040. Determine the exact molarity of a solution of oxalic acid volumetrically.
The Tocris molarity calculator is a useful tool which allows you to calculate the. Once the molecular weight of the solute is known the weight of chemical to dissolve in a solution for a molar solution less than 1M is calculated by the formula. Practice calculations for molar concentration and mass of solute.
Determine the molarity of a. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a. Calculate the molarity of the two solutions.
We have to determine the molarity of a solution of ethanol in water. How to Calculate Normality of a Chemical Solution. This is a great chemistry tool for those who need to determine a solutions molar concentration from the mass volume and molecular weight.
Calculate the molality of a solution if it has a molarity of 185 molkg and the density of solvent is 025 kgm3. C is the molar concentration in molL Molar or M. A 150 M B 118 M C 0130 M D 0768M E 230 M.
The first solution contains 0650 mol of NaOH in 295 L of solution. Molarity is the concentration of a solution or. Determine the molarity of a solution made by dissolving 673 g of Sr ClO 3 2 in enough water to make 5000 mL of solution.
To dilute a solution of known molarity please use the Solution. The second solution contains 215 g of NaCl in 785 mL. Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution.
There are four components in the equation.

Molarity Chemistry Tutorial Youtube

How To Calculate Molarity Using Solute Mass Chemistry Study Com

Molarity Practice Problems Youtube

Question Video Calculating The Molarity Of A Solution From Mass And Volume Nagwa
0 Response to "Determine the Molarity of a Solution"
Post a Comment